How Does Pregnancy Begin Apex
| Mitral stenosis | |
|---|---|
 | |
| Mitral stenosis with marked thickening of the leaflets and left atrial hypertrophy. Superior view. Autopsy preparation. | |
| Specialty | Cardiology |
| Symptoms |
Tardily Stage:
|
| Causes | rheumatic fever, rheumatic eye disease |
| Diagnostic method | Physical Exam, Breast X-ray, Echocardiography, Electrocardiography |
| Treatment | Mitral valve replacement, mitral valvuloplasty |
Mitral stenosis is a valvular centre disease characterized past the narrowing of the opening of the mitral valve of the center.[1] It is most always caused by rheumatic valvular center disease. Normally, the mitral valve is virtually five cm2 during diastole. Whatsoever decrease in area below 2 cm2 causes mitral stenosis. Early on diagnosis of mitral stenosis in pregnancy is very important as the heart cannot tolerate increased cardiac output demand as in the case of exercise and pregnancy. Atrial fibrillation is a common complication of resulting left atrial enlargement, which can lead to systemic thromboembolic complications like stroke.[2]
Signs and symptoms [edit]

Illustration of mitral stenosis, with close-upwards on mitral valve
Signs and symptoms of mitral stenosis include the post-obit:
- Heart failure symptoms, such every bit dyspnea on exertion, orthopnea and paroxysmal nocturnal dyspnea (PND)[three]
- Palpitations[3]
- Chest hurting[3]
- Hemoptysis[iii]
- Thromboembolism[3] in later stages when the left atrial volume is increased (i.east., dilation). The latter leads to increase risk of atrial fibrillation, which increases the risk of blood stasis (motionless). This increases the risk of coagulation.
- Ascites and edema and hepatomegaly (if correct-side heart failure develops)[3]
Fatigue and weakness increase with exercise and pregnancy.[3]
Natural history [edit]
The natural history of mitral stenosis secondary to rheumatic fever (the near common cause) is an asymptomatic latent phase post-obit the initial episode of rheumatic fever. This latent period lasts an average of 16.3 ± 5.2 years. In one case symptoms of mitral stenosis brainstorm to develop, progression to severe disability takes 9.2 ± 4.3 years.[ citation needed ]
In individuals having been offered mitral valve surgery but refused, survival with medical therapy alone was 44 ± 6% at 5 years, and 32 ± 8% at 10 years subsequently they were offered correction.[four]
Cause [edit]

Most all cases of mitral stenosis are due to disease in the middle secondary to rheumatic fever and the consequent rheumatic eye affliction.[three] [v] Uncommon causes of mitral stenosis are calcification[six] [vii] of the mitral valve leaflets, and as a form of congenital centre disease. Nonetheless, in that location are primary causes of mitral stenosis that emanate from a scissure mitral valve.[ citation needed ] Information technology is the well-nigh mutual valvular eye affliction in pregnancy.[8]
Other causes include infective endocarditis where the vegetations may favor increase risk of stenosis. Other rare causes include mitral annular calcification, endomyocardial fibroelastosis, malignant carcinoid syndrome, systemic lupus erythematosus, whipple disease, fabry disease, and rheumatoid arthritis.[9] hurler' affliction, hunter's disease, amyloidosis.
Pathophysiology [edit]

Intracardiac pressure measurements in an private with severe mitral stenosis. Pressure tracings in the left atrium (LA) and the left ventricle (LV) in an private with severe mitral stenosis. Bluish areas represent the diastolic pressure gradient due to the stenotic valve.
The normal surface area of the mitral valve orifice is about 4 to half-dozen cm2. In normal cardiac physiology, the mitral valve opens during left ventricular diastole, to allow blood to flow from the left atrium to the left ventricle. A normal mitral valve will not impede the menstruum of blood from the left atrium to the left ventricle during (ventricular) diastole, and the pressures in the left atrium and the left ventricle during ventricular diastole will be equal. The upshot is that the left ventricle gets filled with blood during early ventricular diastole, with only a pocket-size portion of extra blood contributed by contraction of the left atrium (the "atrial kick") during late ventricular diastole.[ citation needed ]
When the mitral valve expanse goes beneath two cm2, the valve causes an impediment to the flow of blood into the left ventricle, creating a force per unit area gradient across the mitral valve. This gradient may exist increased past increases in the heart rate or cardiac output. Every bit the gradient across the mitral valve increases, the corporeality of time necessary to fill up the left ventricle with blood increases. Somewhen, the left ventricle requires the atrial kick to fill with claret. As the centre rate increases, the amount of fourth dimension that the ventricle is in diastole and can fill up upwards with claret (called the diastolic filling catamenia) decreases. When the heart charge per unit goes above a certain point, the diastolic filling period is insufficient to fill the ventricle with blood and pressure builds up in the left atrium, leading to pulmonary congestion.[ commendation needed ]
When the mitral valve expanse goes less than 1 cm2, there will be an increment in the left atrial pressures (required to push blood through the stenotic valve). Since the normal left ventricular diastolic pressures is most v mmHg, a pressure level gradient beyond the mitral valve of twenty mmHg due to astringent mitral stenosis will cause a left atrial pressure of almost 25 mmHg. This left atrial pressure level is transmitted to the pulmonary vasculature and causes pulmonary hypertension. Pulmonary capillary pressures in this level crusade an imbalance between the hydrostatic pressure and the oncotic pressure, leading to extravasation of fluid from the vascular tree and pooling of fluid in the lungs (congestive heart failure causing pulmonary edema).[ citation needed ]
The constant pressure level overload of the left atrium will crusade the left atrium to increase in size. Equally the left atrium increases in size, it becomes more prone to develop atrial fibrillation (AF). When atrial fibrillation develops, the atrial boot is lost (since it is due to the normal atrial contraction).[ citation needed ]
In individuals with severe mitral stenosis, the left ventricular filling is dependent on the atrial kicking. The loss of the atrial kick due to atrial fibrillation ( i.east. blood cannot flow into the left ventricle thus accumulating in the left atrium ) can cause a sharp subtract in cardiac output and sudden congestive heart failure.[ citation needed ]
Patients with mitral stenosis prompts a series of hemodynamic changes that oft cause deterioration of the patient'south clinical condition. A reduction in cardiac output, associated with dispatch of eye rate and shortening of the diastolic time, frequently leads to congestive heart failure. In addition, when AF sets in, systemic embolization becomes a real danger.[10]
Mitral stenosis typically progresses slowly (over decades) from the initial signs of mitral stenosis to NYHA functional grade 2 symptoms to the development of atrial fibrillation to the development of NYHA functional class Iii or IV symptoms. Once an individual develops NYHA course III or IV symptoms, the progression of the affliction accelerates and the patient'due south condition deteriorates.[ citation needed ]
Diagnosis [edit]
Physical examination [edit]
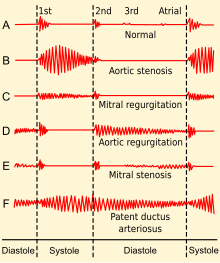
Phonocardiograms from normal and aberrant heart sounds
Upon auscultation of an private with mitral stenosis, the starting time heart sound is usually loud and may be palpable (tapping apex beat) because of increased force in closing the mitral valve. The showtime heart sound is made by the mitral and tricuspid heart valves closing. These are ordinarily synchronous, and the sounds are termed M1 and T1, respectively. M1 becomes louder in mitral stenosis. It may exist the most prominent sign.[three]
If pulmonary hypertension secondary to mitral stenosis is severe, the P2 (pulmonic) component of the second heart sound (S2) volition become loud.[ citation needed ]
An opening snap that is a high-pitch additional sound may exist heard afterward the A2 (aortic) component of the 2d middle sound (S2), which correlates to the forceful opening of the mitral valve. The mitral valve opens when the force per unit area in the left atrium is greater than the pressure in the left ventricle. This happens in ventricular diastole (after closure of the aortic valve), when the pressure in the ventricle precipitously drops. In individuals with mitral stenosis, the force per unit area in the left atrium correlates with the severity of the mitral stenosis. Equally the severity of the mitral stenosis increases, the pressure in the left atrium increases, and the mitral valve opens earlier in ventricular diastole.[3]
A mid-diastolic rumbling murmur with presystolic accentuation will be heard after the opening snap.[iii] [xi] The murmur is best heard at the apical region and is not radiated. Since information technology is a depression-pitch audio, it is heard best with the bell of the stethoscope.[iii] Its duration increases with worsening disease.[3] Rolling the patient toward left likewise as isometric practice will accentuate the murmur. A thrill might exist present when palpating at the apical region of the precordium.[ citation needed ]
Advanced disease may present with signs of right-sided middle failure such as parasternal heave, jugular venous amplification, hepatomegaly, ascites and/or pulmonary hypertension, the latter oftentimes presenting with a loud P2.[3]
Nearly all signs increase with exercise and pregnancy.[iii]
Other peripheral signs include:
- Malar affluent - due to back pressure and buildup of carbon dioxide (CO2). CO2 is a natural vasodilator.[12]
- Atrial fibrillation - irregular pulse and loss of 'a' moving ridge in jugular venous force per unit area
- Left parasternal heave - presence of correct ventricular hypertrophy due to pulmonary hypertension
- Tapping apex shell that is not displaced
Medical signs of atrial fibrillation include:[ citation needed ]
Middle rate is most 100-150/min. Irregularly irregular pulse with a pulse deficit>10. Varying first eye audio intensity. Opening snap is not heard sometimes. Absent a waves in the neck veins. Presystolic accentuation of diastolic murmur disappears. Embolic manifestations may announced.[ citation needed ]
Associated lesions [edit]
With severe pulmonary hypertension, a pansystolic murmur produced by functional tricuspid regurgitation may exist audible along the left sternal border. This murmur is usually louder during inspiration and diminishes during forced expiration (Carvallo's sign). When the cardiac output is markedly reduced in MS, the typical auscultatory findings, including the diastolic rumbling murmur, may not be detectable (silent MS), merely they may reappear as compensation is restored. The Graham Steell murmur of pulmonary regurgitation, a high-pitched, diastolic, decrescendo blowing murmur along the left sternal border, results from dilation of the pulmonary valve band and occurs in patients with mitral valve disease and astringent pulmonary hypertension. This murmur may be duplicate from the more common murmur produced by aortic regurgitation (AR), although it may increase in intensity with inspiration and is accompanied past a loud and often palpable P2. [xiii]
Echocardiography [edit]
| Degree of mitral stenosis | Mean slope | Mitral valve area |
|---|---|---|
| Progressive mitral stenosis | <5 mmHg | >i.five cmtwo |
| Severe mitral stenosis | v–10 mmHg | one.0–ane.5 cm2 |
| Very severe mitral stenosis | > 10 mmHg | < 1.0 cmii |
In most cases, the diagnosis of mitral stenosis is well-nigh easily made by echocardiography, which shows left atrial enlargement, thick and calcified mitral valve with narrow and "fish-mouth"-shaped orifice and signs of correct ventricular failure in advanced disease.[3] It can also show decreased opening of the mitral valve leaflets, and increased blood flow velocity during diastole. The trans-mitral gradient equally measured by Doppler echocardiography is the aureate standard in the evaluation of the severity of mitral stenosis.[ commendation needed ]
Cardiac sleeping room catheterization [edit]
Some other method of measuring the severity of mitral stenosis is the simultaneous left and right heart chamber catheterization. The right middle catheterization (unremarkably known as Swan-Ganz catheterization) gives the doctor the mean pulmonary capillary wedge force per unit area, which is a reflection of the left atrial force per unit area. The left centre catheterization, on the other hand, gives the pressure in the left ventricle. Past simultaneously taking these pressures, it is possible to determine the gradient between the left atrium and left ventricle during ventricular diastole, which is a marker for the severity of mitral stenosis. This method of evaluating mitral stenosis tends to overestimate the degree of mitral stenosis, however, because of the time lag in the pressure tracings seen on the right-heart catheterization and the boring Y descent seen on the wedge tracings. If a trans-septal puncture is made during right heart catheterization, even so, the force per unit area gradient can accurately quantify the severity of mitral stenosis.[ commendation needed ]
Other techniques [edit]
Chest X-ray may also assist in diagnosis, showing left atrial enlargement.[3]
Electrocardiography may show P mitrale, that is, broad, notched P waves in several or many leads with a prominent late negative component to the P wave in lead Vi, and may also exist seen in mitral regurgitation, and, potentially, any cause of overload of the left atrium.[fourteen] Thus, P-sinistrocardiale may be a more appropriate term.[14]
Treatment [edit]
Handling is not necessary in asymptomatic patients.[3]
The treatment options for mitral stenosis include mitral valve replacement past surgery, and percutaneous mitral valvuloplasty by balloon catheter.[15]
The indication for invasive handling with either a mitral valve replacement or valvuloplasty is NYHA functional form Iii or Iv symptoms.[ citation needed ]
Some other option is balloon dilatation.[sixteen] To determine which patients would benefit from percutaneous airship mitral valvuloplasty, a scoring system has been developed. Scoring is based on 4 echocardiographic criteria: leaflet mobility, leaflet thickening, subvalvular thickening, and calcification. Individuals with a score of ≥ 8 tended to have suboptimal results.[17] Superb results with valvotomy are seen in individuals with a crisp opening snap, score < 8, and no calcium in the commissures.[ citation needed ]
Treatment too focuses on concomitant conditions often seen in mitral stenosis:
- Any angina is treated with short-acting nitrovasodilators, beta-blockers and/or calcium blockers[11]
- Any hypertension is treated aggressively, but caution must be taken in administering beta-blockers[xi]
- Whatsoever heart failure is treated with digoxin, diuretics, nitrovasodilators and, if not contraindicated, cautious inpatient administration of ACE inhibitors[xi]

Illustration of mitral valvuloplasty
Mitral valvuloplasty [edit]
Mitral valvuloplasty is a minimally invasive therapeutic procedure to correct an simple mitral stenosis by dilating the valve using a balloon. Under local anaesthetic, a catheter with a special airship is passed from the right femoral vein, upwardly the inferior vena cava and into the right atrium. The interatrial septum is punctured and the catheter passed into the left atrium using a "trans-septal technique." The balloon is sub-divided into 3 segments and is dilated in 3 stages. Commencement, the distal portion (lying in the left ventricle) is inflated and pulled against the valve cusps. Second, the proximal portion is dilated, in order to fix the centre segment at the valve orifice. Finally, the primal department is inflated, this should accept no longer than 30 seconds, since total aggrandizement obstructs the valve and causes congestion, leading to circulatory arrest and flash pulmonary edema.[ citation needed ]
With careful patient pre-selection, percutaneous balloon mitral valvuloplasty (PBMV) is associated with skillful success rates and a low rate of complications. Past far the most serious adverse event is the occurrence of acute severe mitral regurgitation. Severe mitral regurgitation usually results from a tear in one of the valve leaflets or the subvalvular appliance. It can lead to pulmonary edema and hemodynamic compromise, necessitating urgent surgical mitral valve replacement.[ citation needed ]
Other serious complications with PBMV ordinarily relate to the technique of trans-septal puncture (TSP). The platonic site for TSP is the region of the fossa ovalis in the inter-atrial septum. Occasionally, nevertheless, the sharp needle used for TSP may inadvertently traumatize other cardiac structures, leading to cardiac tamponade or serious blood loss.[ citation needed ]
Although the immediate results of PBMV are often quite gratifying, the procedure does not provide permanent relief from mitral stenosis. Regular follow-up is mandatory, to detect restenosis. Long-term follow-upwardly data from patients undergoing PBMV indicates that up to 70–75% individuals can be free of restenosis 10 years following the procedure. The number falls to virtually forty% 15 years mail service-PBMV.[18]
References [edit]
- ^ Carabello, B. A. (2005). "Modernistic Management of Mitral Stenosis". Circulation. 112 (3): 432–7. doi:10.1161/CIRCULATIONAHA.104.532498. PMID 16027271.
- ^ Davidson, Stanley (2014). Principles and Practice of Medicine. Churchill Livingstone. p. 616. ISBN9780702050473.
- ^ a b c d e f g h i j thousand fifty m northward o p q r Chapter one: Diseases of the Cardiovascular organization > Section: Valvular Middle Disease in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Footstep-Up to Medicine (Pace-Upward Series) . Hagerstwon, Doctor: Lippincott Williams & Wilkins. ISBN978-0-7817-7153-v.
- ^ "Los xiii mejores consejos para dejar de fumar". cursoenarm.net. Archived from the original on 2016-11-13.
- ^ "Mitral Stenosis: Center Valve Disorders: Merck Manual Home Edition". Archived from the original on 2009-03-02. Retrieved 2009-03-14 .
- ^ Bertazzo, S. et al. Nano-belittling electron microscopy reveals key insights into man cardiovascular tissue calcification. Nature Materials 12, 576-583 (2013).
- ^ Miller, J. D. Cardiovascular calcification: Orbicular origins. Nature Materials 12, 476-478 (2013).
- ^ Gelson, E; Gatzoulis, M; Johnson, M (2007). "Valvular heart disease". BMJ. 335 (7628): 1042–5. doi:10.1136/bmj.39365.655833.AE. PMC2078629. PMID 18007005.
- ^ Bonow, Robert O.; Isle of man, Douglas L.; Zipes, Douglas P.; Peter Libby Grand.D. (2012). Braunwald's Eye Disease: A Textbook of Cardiovascular Medicine. Elsevier Saunders. ISBN978-1-4377-0398-half dozen.
- ^ American Heart Journal[ total commendation needed ]
- ^ a b c d VOC=VITIUM ORGANICUM CORDIS, a compendium of the Department of Cardiology at Uppsala Academic Infirmary. By Per Kvidal September 1999, with revision by Erik Björklund May 2008
- ^ "Mitral Stenosis: Valvular Disorders: Merck Manual Professional". Merckmanuals.com. Archived from the original on 2014-08-19. Retrieved 2013-02-21 .
- ^ Kasper, Dennis 50.; Fauci, Anthony S.; Hauser, Stephen L.; Longo, Dan L.; Larry Jameson, J.; Loscalzo, Joseph (half dozen Feb 2018). Harrison's principles of internal medicine (20th ed.). p. 1815. ISBN978-one-25-964404-7.
- ^ a b medilexicon.com < P mitrale Archived 2011-11-03 at the Wayback Motorcar Citing. Stedman's Medical Dictionary. Copyright 2006
- ^ "Mitral Stenosis". The Lecturio Medical Concept Library . Retrieved 11 August 2021.
- ^ Wilkins, G T; Weyman, A E; Abascal, Five M; Block, P C; Palacios, I F (1988). "Percutaneous balloon dilatation of the mitral valve: An analysis of echocardiographic variables related to outcome and the mechanism of dilatation". Heart. 60 (iv): 299–308. doi:ten.1136/hrt.60.4.299. PMC1216577. PMID 3190958.
- ^ Abascal, 5. One thousand.; Wilkins, G. T.; O'Shea, J. P.; Choong, C. Y.; Palacios, I. F.; Thomas, J. D.; Rosas, E.; Newell, J. B.; et al. (1990). "Prediction of successful upshot in 130 patients undergoing percutaneous balloon mitral valvotomy". Apportionment. 82 (two): 448–56. doi:x.1161/01.CIR.82.2.448. PMID 2372892.
- ^ Fawzy, ME; Shoukri, M; Al Buraiki, J; Hassan, W; El Widaal, H; Kharabsheh, S; Al Sanei, A; Canver, C (2007). "Seventeen years' clinical and echocardiographic follow upward of mitral balloon valvuloplasty in 520 patients, and predictors of long-term outcome". The Journal of Centre Valve Disease. 16 (5): 454–60. PMID 17944115.
External links [edit]
How Does Pregnancy Begin Apex,
Source: https://en.wikipedia.org/wiki/Mitral_stenosis
Posted by: freyfraidgetefe.blogspot.com


0 Response to "How Does Pregnancy Begin Apex"
Post a Comment